A second life for CO2
- TU News

It is already almost too late: In order to observe the climate target of limiting global warming to 1.5 °C compared to the pre-industrial period, we have already released too much carbon dioxide into the atmosphere. So it is no longer enough to massively cutCO2 emissions or reduce them to zero with immediate effect: We need to removeCO2 from the atmosphere again. This is the conclusion reached by the Intergovernmental Panel on Climate Change in a special report published in 2019.
But how canCO2 be captured from the air? And how do you prevent it from re-entering the atmosphere after a certain time? This is what Professor David Agar from the Faculty of Bio and Chemical Engineering at TU Dortmund University is researching. "In nature, plants bindCO2 in biomass," he explains. "But that's only a temporary solution: when the plants rot, theCO2 is released again."
Process cleverly combined
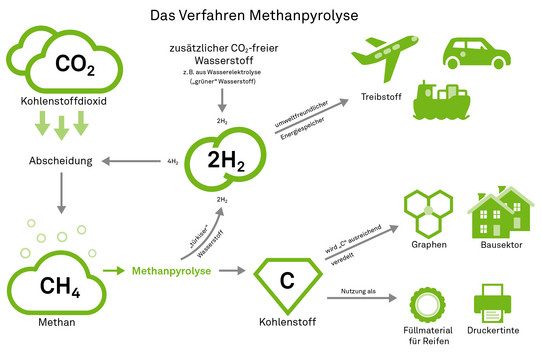
Agar therefore relies on a chemical approach. To do this, he first uses solid adsorbents known as amines, which react with theCO2 from the air and bind it to themselves. In order to reuse the material, the bound carbon dioxide must be removed again. "This process requires high temperatures, so a lot of energy," Agar says. "To avoid harming the climate, only renewable energy can be used for this." His team uses a clever combination to generate the heat it needs itself while also processing theCO2 filtered from the air. "We let theCO2 react with hydrogen to form methane, or CH4. This is synthetic natural gas," he explains. "This reaction releases a lot of heat, which we can use to regenerate the amines. So thermodynamically, it makes sense to couple the processes." Currently, his team is working on making this process more efficient.
The next question in the research process is: What becomes of the methane produced? "You could simply burn it as natural gas," explains Agar. "That would beCO2-neutral, after all, because we have previously filtered theCO2 that is produced during combustion out of the air. But ultimately, we want to achieve a negativeCO2 balance." One possibility would be to make the methane available to the chemical industry. Here it serves as a starting material for many chemical compounds. "But these would also decompose again at some point - similar to biomass - and theCO2 would be released again," Agar describes.
Final storage or further processing?
He therefore considers geological processes to be a long-term solution. These aim to permanently storeCO2 from the air in the ground. In this case, it would not necessarily have to be processed into methane first. Some technologies, in whichCO2 is injected at high pressure into underground reservoirs, such as old gas fields, involve risks, including the danger that theCO2 will escape again or that changes in pressure will trigger earthquakes. As a result, such processes are no longer under discussion in Germany. Another option is to injectCO2 into porous basalt rock. This rock reacts with theCO2 to form carbonates, or minerals. The reaction proceeds slowly, but offers a safe and permanent form of storage.
Agar itself, however, takes a different approach. His ideal is not just to remove theCO2 from the atmosphere and store it with as little risk as possible - all the components should be put to good use. To this end, he is working on a process called methane pyrolysis: "We want to make hydrogen and elemental carbon out of the methane that we have extracted from the air duringCO2 capture," he explains. In the process, one molecule of CH4 becomes one C (carbon) and two H2 (hydrogen). The hydrogen can be used as an environmentally friendly energy store, for example as fuel for hydrogen cars, airplanes or ships. Alternatively - and here we come full circle - it can be used again in the first step,CO2 capture, to reactCO2 to form methane.
Carbon also has numerous potential uses, for example as a pigment in printer ink. "Right now, it's mainly used by the tire industry as a filler," Agar tells us. "But here the quantities are limited." Should it prove possible to refine the carbon sufficiently, higher-value areas of application would also be open. "It would be interesting to use the extracted carbon to make graphene, the thinnest material in the world, consisting of a single layer of carbon atoms linked together," Agar says. Graphene is 200 times stronger than steel, flexible and can be used as a conductor of heat and electricity. "Carbon fibers could also conceivably be used in the construction sector, for example."
When carbon becomes a problem
But such applications are far in the future. First, there are numerous obstacles to overcome in methane pyrolysis. If methane were burned under normal conditions, it would react with atmospheric oxygen to form water and carbon dioxide. That can't happen in pyrolysis. "As soon as oxygen is involved, you've lost," Agar says. "So we try to decompose the methane in the absence of oxygen." Temperatures above 1,000°C are required for the CH4 molecule to split into its constituent carbon and hydrogen.
One problem here is that while the hydrogen produced rises as a gas, the carbon is deposited - at the hottest spot, of all places, the wall of the pipes. The corresponding systems are therefore soon clogged, and the carbon deposit on the walls reduces the possible heat input. Agar and his team are pursuing various approaches to solving this problem. In a project funded by the German Federal Ministry of Education and Research together with BASF, the researchers have developed a reactor with a so-called carbon moving bed.
Here, carbon continuously trickles down the reactor, to which the newly formed carbon can attach. "In this way, the carbon is transported away and does not settle on the walls," explains Agar.
A second approach aims to avoid the walls altogether. Instead of conveying the heat via the walls, one generates an electric arc, i.e., a plasma fed from electrical energy, in which the splitting of the methane takes place. "This process is flexible to use and can be ramped up and down quickly," Agar says. The downside, however, is that so far it is still very energy-intensive and has poor efficiency.

Out of scientific interest, Agar also devotes himself to a third approach: "We continuously rinse the walls of our reactor with a liquid film of liquid metal," he reports. This causes the carbon to flow off with the metal, leaving the walls free. "This works similarly to the carbon bed, but with liquid instead of solid. This has advantages for heat balance, for example," Agar says. "In both cases, it's good that carbon and hydrogen leave the reactor in different directions, so there are no back reactions."
What's possible, what's profitable?
None of the approaches is yet commercially viable. On an industrial scale, hydrogen has so far been produced primarily from fossil natural gas by reacting it with steam. Since the production of this so-called "gray" hydrogen releasesCO2, it further contributes to the climate problem. But its production is so cheap that it has so far crowded out other climate-friendly technologies. Alternatives would be so-called "blue" hydrogen, which is produced in the same way as "gray" hydrogen but where theCO2 produced is captured and stored so it does not enter the atmosphere, and "green" hydrogen, which is produced by electrolysis of water with renewable electricity and isCO2-free. "But until you penalizeCO2 emissions financially, these technologies are not competitive," Agar says.

The hydrogen from methane pyrolysis that he is researching is called "turquoise" hydrogen. "How profitable this process is also depends on what you do with the resulting carbon," he explains. If it is indeed possible to find high-value applications, it would significantly improve profitability. Currently, however, the carbon produced during pyrolysis is still too inconsistent. "But that's more of a materials engineering research area. We're just providing the carbon," says Agar, who sees the hydrogen itself primarily as a tool on the road to a more environmentally friendly energy supply. "Hydrogen is not a panacea," he says. "It's a good way, with renewable energy, to balance demand and supplybalance demand and supply and to provide longer-term storage."
Hydrogen cars, on the other hand, are too inefficient compared to electric cars. In his view, the situation is different with airplanes and ships that use environmentally friendly hydrogen as fuel. "Because of the long distances to be covered, we rely on chemical energy storage here. In this case, hydrogen could replace fossil fuels."
By combiningCO2 capture and methane pyrolysis, the hydrogen Agar produces is not onlyCO2 neutral, but can actually contribute to the ideal of a negativecarbon footprint. Agar itself describes its process as a transitional technology: "We hope to use it to contribute to the shift to environmentally friendly forms of technology." For new technologies to be worthwhile, however, political measures are needed above all, in addition to scientific advances. The price ofCO2 certificates, in particular, is an important adjusting screw, he said. "We are already past the point where we simply achieve our climate goals by restricting emissions. We need to actively work againstCO2 in the atmosphere," Agar says. "Our technologies can provide a building block for that."
Text: Elena Bernard
About the person
Prof. David W. Agar is Professor of Chemical Engineering in the Department of Biochemical and Chemical Engineering. He studied bioengineering at the University of Wales and received his PhD in chemical engineering from the University of Houston. After a total of sixteen years in industry at BASF, he became Professor of Technical Chemistry at TU Dortmund University in 1997.
His research interests include multifunctional reactors, microreactors, process intensification, and environmental applications of reaction engineering. Together with colleagues from the BCI faculty, he authored the textbook "Introduction to Technical Chemistry", which received the Literature Award of the Chemical Industry Fund in 2011.